Abstract
Chronic kidney disease is observed in up to 50% of adults with sickle cell disease (SCD) and is a consistent predictor for increased morbidity and early mortality. Progression of kidney disease can be manifested by a rapid decline in estimated glomerular filtration rate (eGFR). In retrospective studies, up to 38% of SCD patients have a rapid decline in kidney function, defined from the non-SCD literature as an eGFR slope < -3.0 mL/min/1.73m 2. Clinical and genetic predictors and the appropriate cutoff for rapid eGFR decline in SCD are unclear, but are paramount for guiding intervention studies in sickle cell nephropathy.
We investigated 1) genetic, laboratory, and clinical risk factors for eGFR decline and 2) the rate of eGFR decline that best predicted mortality risk in a longitudinal cohort of SCD patients enrolled in a prospective registry at our institution. Between 10/2009 and 2/2018, 439 SCD patients were recruited. Blood samples, clinical and laboratory data were collected after obtaining consent at the time of enrolment during a clinic visit without the patient being in a vaso-occlusive crisis. 352 SCD patients with > 6 months of outpatient eGFR assessments were included in this analysis. The eGFR slope was calculated for each patient by linear regression of eGFR by time. Genotyping for the APOL1 G1 and G2 kidney risk variants and alpha thalassemia were performed by PCR. High-risk APOL1 status was defined as being either homozygous or compound heterozygous for the G1 and/or G2 variants. The statistical analyses for predictors of eGFR decline were conducted using linear regression, adjusting for age, sex, SCD genotype, hydroxyurea use, angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB) use, and baseline eGFR. Median and interquartile ranges (IQR) are provided.
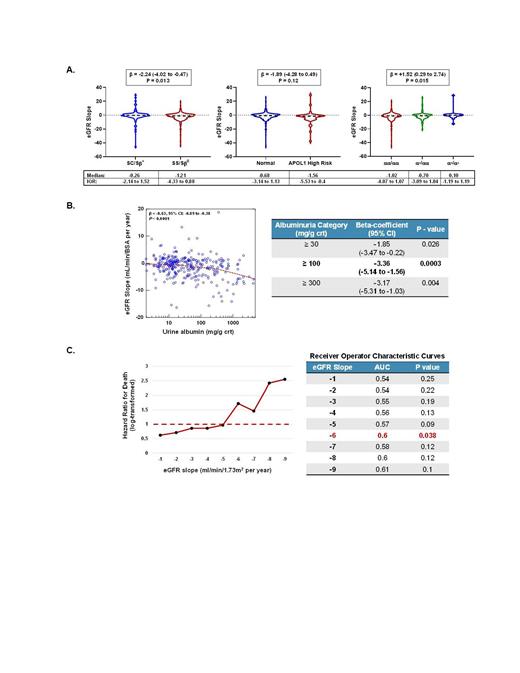
The median age of the cohort was 32 years old (IQR, 24 - 43 years), 60% were female, 76% had Hb SS or Sβ 0-thalassemia genotype, 47% were on hydroxyurea, and 12% were on ACEi or ARB therapy. With a median follow up of 6.7 (IQR, 3.8 - 8.5) years, the median annual eGFR slope was -0.9 (IQR, -3.6 to 1.1) mL/min/1.73m 2. A faster rate of eGFR decline was observed in SCD patients with the Hb SS or Sβ 0-thalassemia genotype, with high-risk APOL1 status, and in those without coinheritance of α-thalassemia (Figure 1A). The urine albumin concentration, based on the average of two consecutive values from the time of enrolment, was significantly associated with a more rapid eGFR decline (β -0.63, P < 0.0001). An albuminuria cutoff of ≥ 100 mg/g creatinine was a stronger predictor for eGFR decline than cutoffs of ≥ 30 or ≥ 300 mg/g (Figure 1B). During the follow up period, we observed 26 deaths (7.4% mortality). An annual eGFR slope of < -6 mL/min/1.73m 2 was independently associated with a greater risk for mortality, after adjusting for age, sex, SCD genotype, hydroxyurea use, ACEi or ARB use, and baseline eGFR (Figure 1C). Using receiver operating curves, this cutoff was also associated with the largest area under the curve for predicting mortality.
Our data highlights genetic risk factors and supports albuminuria as an independent predictor of eGFR decline in a longitudinal cohort of SCD patients. We also demonstrate that an annual eGFR slope of < -6 mL/min/1.73m 2 is the strongest predictor for mortality in our cohort. This threshold will need to be validated in other longitudinal SCD cohorts. The association of urine albumin ≥ 100 mg/g creatinine with eGFR decline supports using this cutoff as a clinical biomarker to identify high risk patients for kidney disease progression and for initiating disease modifying and reno-protective therapies.
Saraf: Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding. Gordeuk: Modus Therapeutics: Consultancy; Novartis: Research Funding; Incyte: Research Funding; Emmaus: Consultancy, Research Funding; Global Blood Therapeutics: Consultancy, Research Funding; CSL Behring: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal